![Poly A Guarantee Service Poly A Guarantee Service]()
mRNA Applied Vector
Ready-to-use mRNA Vector with exceptional
cloning efficiency
and poly(A) stability
Specification
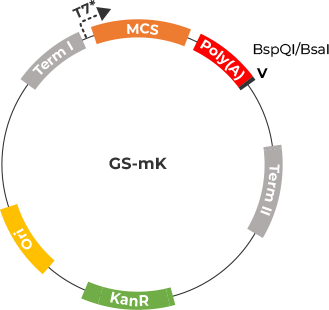
GS-mK Vector
Description:
The GS-MK Vector has been adapted using the pUC57-kana Vector. This involved
removing the Lac promoter sequence, and placing terminator elements both before and after the
MCS. These modifications have improved poly(A) tail stability and cloning efficiency.
| Plasmid Type |
Cloning Vector |
| Derived from |
pUC57-kana |
| Promoter |
N/A |
| Resistance |
Kanamycin
|
| Cloning Method |
Restriction Enzyme/MCS
|
| Poly(A) |
Empty/100A/120A/30+30+43A/31+71A |
| Cleavage Site |
BspQI/BsaI |
GS-CMV Vector
Description:
The GS-CMV Vector has been adapted using the pVAX1 Vector, a distinctive vector
approved by the US FDA for clinical trials and suitable for eukaryotic transfection expression
assays. GS-CMV has also incorporated terminator elements to enhance the stability of the poly(A)
tail and has amplified its plasmid copy number. This ensures a decrease in potential genome
contamination and minimizes the loss of quantity during plasmid extraction.
| Plasmid Type |
Expression Vector |
| Derived from |
pVAX1 |
| Promoter |
CMV |
| Resistance |
Kanamycin
|
| Cloning Method |
Restriction Enzyme/MCS |
| Poly(A) |
Empty/100A/120A/30+30+43A/31+71A |
| Cleavage Site |
BspQI/BsaI |
*Our mRNA vector has been deliberately crafted without a promoter. For more information please refer to the FAQ
Case Study
-
GS-mK Engineered for Superior Cloning Efficiency
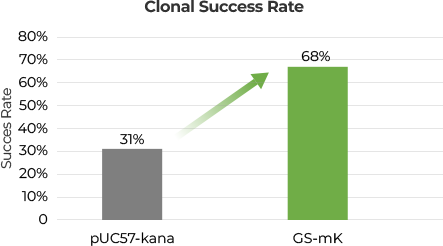
Figure 1. Gene Fragment encompassing a polyadenylate tract of 100 adenosine
residues was independently cloned into pUC57-kana and GS-mK vectors. These recombinant
constructs were subsequently transformed into a competent GenPoly bacterial strain for
propagation. Isolated clones were subjected to molecular screening and sequencing to verify
the integrity of the gene fragments. Clonal efficiency was quantified by assessing the
proportion of clones harboring an intact insert.
-
GS-CMV Engineered for Superior Plasmid Production
Figure 1. The plasmid production capability was evaluated by comparing the
pVAX1 vector with
the GS-CMV vector. Following the transformation of the respective vectors into the competent
GenPoly strain, single colonies were selected and grown in 400 mL of 3xLB medium. The
cultures were then incubated at 37°C for 13 hours to promote the growth of the bacterial
culture. Post incubation, the plasmids were extracted, and the graph illustrates the total
plasmid yield obtained.
FAQ
1. Why does your mRNA vector lack a T7 Promoter?
A: Our mRNA vector has been deliberately crafted without a promoter and UTR regions to allow
users the versatility to customize their experiments with their selected promoter and UTR sequences. To
adapt the vector to your needs, just integrate your chosen promoter and UTRs alongside your ORF during the
cloning process into the MCS region.
2. Can I license the mRNA applied vector for commercial use?
Certainly, we provide a commercial licensing service for clients intending to utilize our mRNA
applied vectors for commercial purposes. Please contact us for further information.
3. Can I request more information on the vectors?
Certainly, we have extensive data in regards to our vector, such as Poly(A) tail stability, in
vitro transcription (IVT) mRNA purity, and IVT mRNA expression levels. For more detailed information, please
get in touch with us.
Relative Services
Tailor and authenticate the polyadenylation of your mRNA template
Learn More
Ideal for scientists seeking high-quality large-scale mRNA IVT template
Learn More
Basic research to animal study grade
Plasmid DNA from microgram to gram level
Poly(A) and AAV ITR guarantee
Learn More
Get in Touch
with GenScript mRNA Applied Vector Service